
Metallic properties include a lustrous appearance, good electrical conductivity, and the ability to form alloys with other metals.
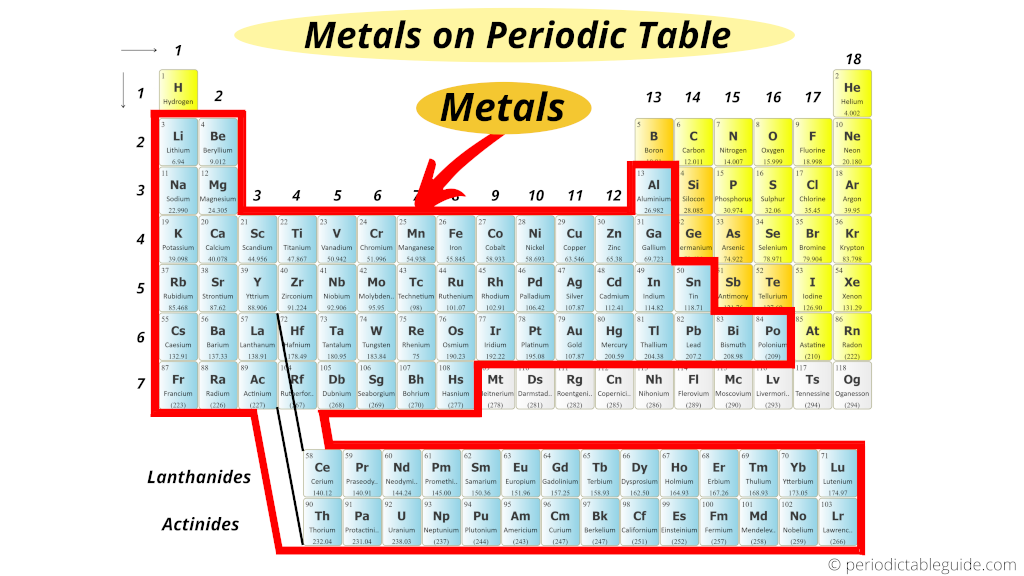
Elements in this group are characterized by their metallic properties. The left column of the periodic table is called the “metals” group. Where are metals located on the periodic table? Metals are located on the left side of the periodic table, and they are characterized by their ability to conduct electricity and heat. This column is called the “metals” group.Įlements in this group are characterized by their metallic properties, which include a lustrous appearance, good electrical conductivity, and the ability to form alloys with other metals. Metals are located in the left column of the periodic table.

Where are the most reactive metals on the periodic table found?.So, elements on the left side of the periodic table are more likely to be metals than those on the right side.Why are metals on the left of the periodic table?.
METALS PERIODIC TABLE HOW TO
How to identify METALS NONMETALS and METALLOIDS on the PERIODIC TABLE.Which group in the periodic table contains metals?.Metals are located on the left side of the periodic table, and they are characterized by their ability to conduct electricity and heat.Where are metals located on the periodic table?.This column is called the “metals” group. Metals are located in the left column of the periodic table.


 0 kommentar(er)
0 kommentar(er)
